Abstract
BACKGROUND: Allogeneic hematopoietic stem cell transplantation (HCT) is a standard treatment for high-risk relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL). However, about 30-40% of patients still relapse after HCT. Chimeric antigen receptor-modified T cell (CAR-T) therapy holds great promise as a salvage approach. Although CD19-targeted CAR-Ts have demonstrated a rapid and significant anti-tumor effect in patients with B-cell malignancies, many still suffer disease relapse due to the decrease or loss of CD19 antigen. Therefore, novel curative approaches are needed.
PURPOSE: The aim of this study is to determine the best timing for applying CAR-T therapy in high-risk B-ALL patients based on high-sensitivity analysis of tumor cells by next-generation sequencing (NGS) detecting minimal residual disease (MRD). An innovative CAR-T therapy concept, CAR2.0, has been proposed, which uses multi-antigen-targeted CAR-Ts to prevent antigen escape and prolong remission. Based on NGS-MRD detection, patients with potential tumor relapse can be identified early and managed accordingly. On the other hand, if extended negative NGS-MRD is found, the patient is deemed at low risk and no further treatment is needed. The study proposes to treat high-risk B-ALL patients in the first remission period with CAR2.0 followed by continued disease monitoring by NGS-MRD to avoid HCT.
METHODS: B-ALL patients who have high tumor burden and high-risk genetic indication is enrolled with the understanding that CAR-T therapy will be applied in the first remission and disease will be continuously monitored by NGS-MRD. The tumor cells from initial diagnosis will be analyzed for target antigens by immunostaining and to detect clonally rearranged immunoglobulin gene sequences by NGS-MRD with sensitivity ≤10-6. Patients will receive 4SCAR19 (CD19scFv-CD28/CD27/CD3ζ-iCasp9) therapy in the first remission and will be followed up for CAR-Ts, B cells and IgG/IgA/IgM in the blood, and bi-monthly NGS-MRD for residual tumor cells in the bone marrow (BM). A booster CAR-T treatment targeting additional tumor antigen such as CD22 will be applied 2 months later. A consolidation CAR-T treatment based on known target antigens will be applied 6 months after the first CAR-T infusion. The cyclophosphamide/fludarabine chemo-conditioning will be included prior to the first CAR-T treatment only. The CAR-T infusion dose is 1-5x106 CAR-Ts/kg. The quality of apheresis cells, efficiency of gene transfer, T cell proliferation, CAR-T infusion dose and blood CAR copies are quantitatively documented. The end point for this study is the establishment of NGS-MRD negativity for 1 year, with continued follow-up for 5 years.
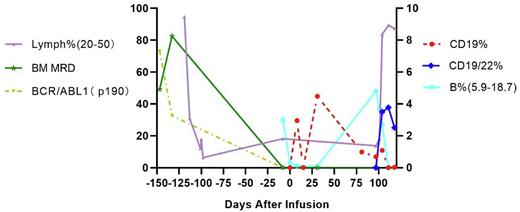
RESULTS: A 20 years old female was diagnosed with BCR/ABL1 P190 positive B-ALL, with 82.52% tumor blasts in the BM. NGS identified two high-frequency IgH clones at 66.29% and 30.41%. The patient received induction chemotherapy (VTCP, CAM, VP+MTX) combined with TKI therapy (Nilotinib) and achieved complete remission (CR) after the first round of treatment based on both flow cytometry and NGS MRD analyses. During the fourth round of chemotherapy (Methotrexate-Cytarabine-Dexamethasone), the patient suffered severe oral and intestinal mucositis, along with kidney and liver toxicity and required week-long dialysis. A decision was made to stop chemotherapy and start the first 4SCAR19 therapy of 3.53x106 CAR-Ts/kg, while maintaining the TKI therapy. The CAR-T cells in blood, the B cell count and Ig levels were monitored, and a booster CD22/CD19 dual CAR-Ts (4SCAR2219) was given at a dose of 3.35 x106 CAR-Ts/kg on day 96 (see figure). Consistent with 4SCART safety profile, the patient experienced no greater than grade 1 CRS. NGS-MRD detection of tumor cell clones remained negative on day 96 after the first CAR-T infusion and at 6 months into the remission. In summary, the 4SCAR2.0 therapy regimen appeared safe and effective in the innovative NGS-MRD/4SCAR2.0 B-ALL study regimen with in vivo persistency for more than three months. The simultaneous expansion of CD19 mono-specific and CD22/CD19 bi-specific CAR-Ts supports a stable maintenance treatment with good life quality as compared with conventional chemotherapy.
CONCLUSIONS: The combination of 4SCAR2.0 with NGS-MRD detection of tumor clones in the management of high-risk B-ALL appears to be safe and may be a logical option for patients not elected for HCT therapy.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal